
Melting point The temperature at which the solidliquid phase change occurs. Exposure to cobalt-60, a powerful gamma ray emitter, may cause cancer. Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Cobalt should be handled with care because of its toxicity and its risk factor in nuclear confrontation. As stated, you could simply count the boxes on the periodic table, and since Cobalt is the 7th element of the first row transition metals, we get Co: Ar 4s 2 3d 7.

Check for configuration drift routinely to identify resources that were. Hunting and detecting Cobalt Strike Cobalt Strike. The reason why it is 3d 7 can be explained using the periodic table. For breaches involving electronic health information, you may need to notify the. CobaltStrikeScan: Scan files or process memory for CobaltStrike beacons and parse their configuration. The Aufbau principle predicts that the 4s orbital is always filled before the 3d orbitals, but this is actually not true for most elementsFrom Sc on, the 3d orbitals are actually lower in energy than the 4s orbital, which means that electrons enter the 3d orbitals first. In small amounts, cobalt is an essential element for humans and many other living organisms, and it is also a central component of vitamin B-12 or cobalamin. Thus, the electron configuration for Cobalt at ground state would simply be Co: Ar 4s 2 3d 7. Electron Configuration and Oxidation States of Cobalt Electron configuration of Cobalt is Ar 3d7 4s2. What is the electron configuration for the calcium (Ca) atom in its This problem has been solved You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Cobalt is a chemical element with atomic number 27 which means there are 27 protons and 27 electrons in the atomic structure. Brandt between 17 when he was able to show that cobalt colors glass a rich blue. What is the electron configuration for the cobalt (Co) atom in its ground state a. So, through this diagram the configuration of cobalt is, 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3. The Aufbau diagram that shows filling is, The arrows denote the manner of filling.
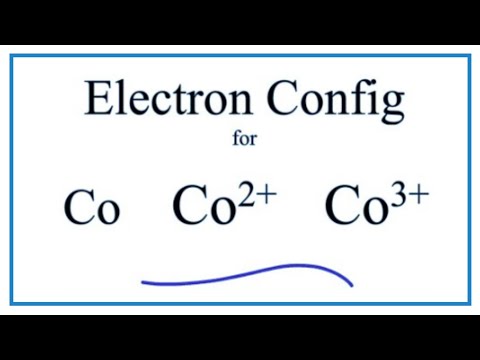
The electronic configuration of cobalt will consist of filling 27 electrons according to the Aufbau principle. The chemistry of the lanthanides is dominated by the +3 oxidation. This solid ferromagnetic silver-white element was known in ancient times for its compounds, but its discovery was credited to G. We have been given cobalt which has atomic number z 27. The electronic structure of the lanthanide elements, with minor exceptions, is Xe6s24fn. Primary XPS region: Co2p Overlapping regions: Co LMM, Ba3d Binding energies of common chemical states: Chemical state Binding energy Co2p 3/2 Co metal: 778. Many studies have examined the immunocytochemical localization of GlyRs at the light and electron microscopic levels using generic GlyR -subunit monoclonal. Exposure to cobalt-60, a powerful gamma ray emitter, may cause cancer.Obtained from: arsenic, oxygen, sulfur, cobatineįrequently, cobalt is associated with nickel because both elements have characteristic ingredients of meteoric iron. Cobalt X-ray photoelectron spectra, cobalt electron configuration, and other elemental information. In small amounts, cobalt is an essential element for humans and many other living organisms, and it is also a central component of vitamin B-12 or cobalamin. Brandt between 17 when he was able to show that cobalt colors glass a rich blue. This solid ferromagnetic silver-white element was known in ancient times for its compounds, but its discovery was credited to G. Obtained from: arsenic, oxygen, sulfur, cobatineįrequently, cobalt is associated with nickel because both elements have characteristic ingredients of meteoric iron.


 0 kommentar(er)
0 kommentar(er)
